Abstract
Background: Relapse of acute myeloid leukemia (AML) following allogeneic hematopoietic cell transplantation (allo-HCT) has historically been associated with dismal prognosis with most patients failing to achieve subsequent remission and estimated 2-year overall survival (OS) of <15 %. Rapid progress in the understanding of AML biology and genomics resulted in recent FDA approval of several novel small molecules and immunotherapies for the treatment of front-line and relapsed/refractory AML, as early as 2017. We hypothesized that the availability of novel therapies for AML relapsing after allo-HCT has resulted in an improved OS in recent years.
Methods: Patients with AML ≥18 years old who underwent allo-HCT at Stanford University between the years 2010 and 2021 and subsequently relapsed were included in this study. Patients were divided into two eras based on year of relapse: 2010-2016 (early era) and 2017- 2022 (later era). Descriptive statistics were used to characterize patients across the two cohorts; Kaplan Meier method was used to evaluate OS.
Results: A total of 258 adults with AML relapsed following allo-HCT; 142 (55%) transplanted in the early era and 116 (45%) in the later era. Relative to the early era, patients who relapsed in the later era were more likely to be Hispanic (19.8% vs. 8.5%, P=0.01), have adverse risk leukemia (64.7% vs 52.1%, P=0.039), poorer performance status as defined by KPS <90 (46.6% vs. 16.9%, p<0.001), an HCT-comorbidity index of ≥ 3 (43.1% vs. 28.2%, P=0.012), and receive alternative donor transplant (haploidentical donors 11.2% vs 3.5%, P=0.002; umbilical cord blood 9.5% vs. 4.2%, P=0.002). Post-HCT maintenance therapy was used more frequently in the later era (16.4% vs. 2.1%, P=0.002). Median age, sex, and conditioning intensity did not significantly differ between the two cohorts.
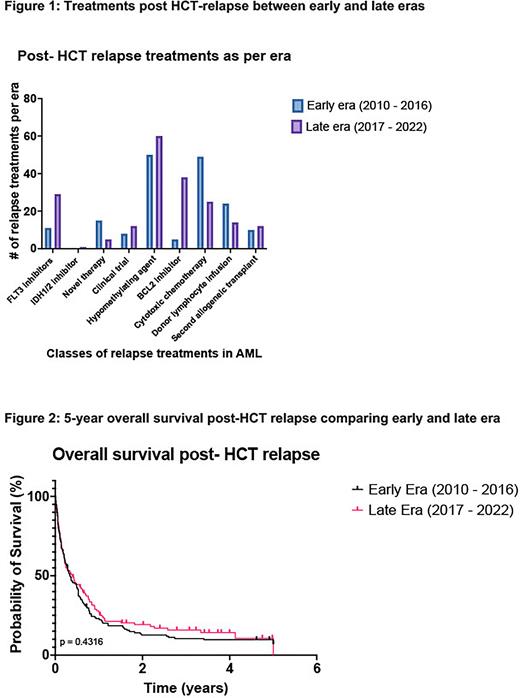
The median time to AML relapse post-HCT was similar in the early vs late era (149 days vs. 172 days, P=0.003). The management of relapse differed significantly across eras (Figure 1), as expected, with patients in the later era more commonly receiving targeted therapy with FLT3 inhibitors and IDH1/2 inhibitors (25% vs. 7.7%), hypomethylating agents (HMA) (51.7% vs. 35.2%) and BCL2 inhibitors (32.8% vs 3.5%) and less likely receiving cytotoxic chemotherapy (21.6% vs 34.5%). The use of donor lymphocyte infusion increased over time (16.9% vs. 12.1%) as did second allo-HCT (10.3% vs. 7%).
Despite increased use of novel therapies in the later era, there was no improvement in median OS compared to the early era (5.1 months versus 4.5 months, p=0.43) or 2-year OS following post-HCT relapse (19.1% vs. 12.4%, P=0.26), (Figure 2). Across both eras, relapsed AML was the most common cause of death in this cohort, attributed to 83.7% of all deaths.
Conclusion: Despite the greater use of novel therapies to treat post-HCT relapse of AML in recent years, OS remains poor and has not improved. Relapsed AML following allo-HCT remains a significant clinical challenge despite advances in modern AML therapeutics.
Disclosures
Lowsky:Orca Bio: Research Funding. Arai:Kadmon: Membership on an entity's Board of Directors or advisory committees. Meyer:indee labs: Membership on an entity's Board of Directors or advisory committees; Orca Bio: Research Funding; GigaGen: Other: Co-founder, scientific advisor; Triursus Therapeutics: Other: Co-founder, scientific advisor. Negrin:CoImmune: Current equity holder in private company, Current holder of stock options in a privately-held company; University of Pennsylvania: Other: DSMB or Advisory Board; Novartis: Consultancy; UptoDate: Honoraria; Amgen: Consultancy; Kuur: Consultancy; Garuda: Consultancy; Magenta: Consultancy, Current equity holder in publicly-traded company; BioEclipse Therapeutics: Current equity holder in private company, Current holder of stock options in a privately-held company. Shiraz:Kite, a Gilead company: Research Funding. Shizuru:Jasper Therapeutics: Consultancy, Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees; rBio: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees; Shoreline BioSciences: Current equity holder in private company, Honoraria, Membership on an entity's Board of Directors or advisory committees. Sidana:Oncopeptides: Consultancy; Sanofi: Consultancy; Janssen: Consultancy, Research Funding; Allogene: Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; Magenta Therapeutics: Consultancy, Research Funding; Prothena: Honoraria. Frank:Roche/Genentech - Wife: Current equity holder in private company, Current holder of stock options in a privately-held company; Allogene Therapeutics: Research Funding; Kite/Gilead: Honoraria, Research Funding; Adaptive Biotechnologies: Consultancy, Honoraria, Research Funding. Miklos:Fosun Kite: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Kite, a Gilead Company: Research Funding; Adaptive Biotech: Consultancy; Bristol Meyers Squibb: Consultancy; Novartis: Consultancy; Allogene: Research Funding; Pharmacyclics: Patents & Royalties: cGVHD Ibrutinib patent .
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal